Copyright ©2017 all rights reserved
Designed by Plethora Themes

Directed Remote Metalations on larger PAHs
University of Stavanger, Norway
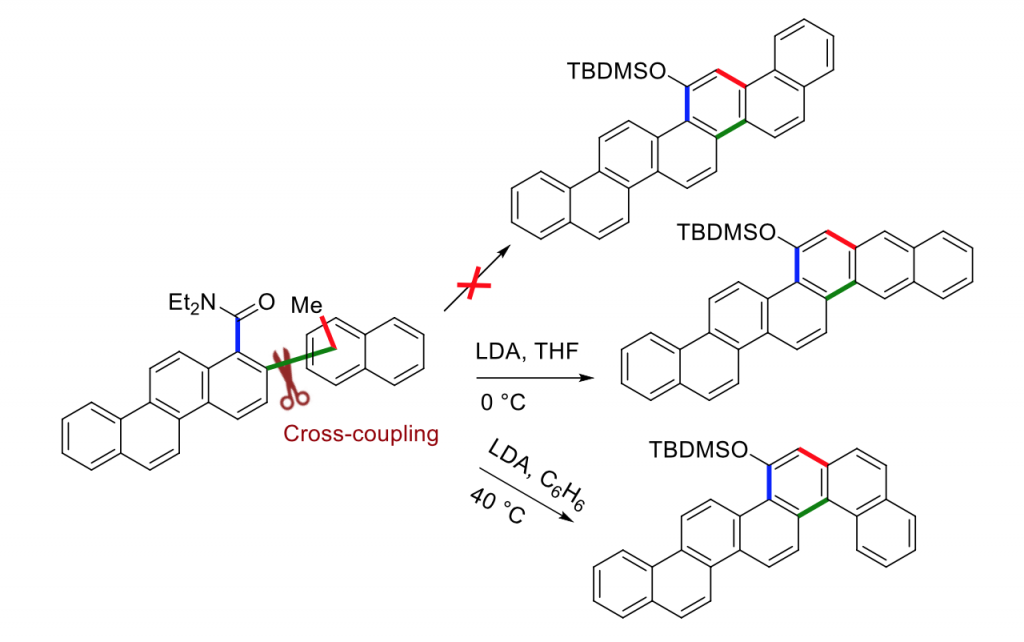
Directed metalations like DoM (Directed ortho metalation) and DreM (Directed remote metalations)1 using an organolithium base are often regioselective and efficient in functionalising the C─H bonds near a directing metalation group (DMG). DreM has been reported to synthesize fluorenones and phenanthrenes2 that are an integral part of many important natural products. This methodology has a great but so far unexplored potential for making specific larger PAHs. Our lab has been studying the reactivity pattern of DreM using cross-coupled substrates bearing ortho-methylnaphthalenes. DreM in such substrates can undergo metalation at the remote position on the aromatic ring forming fluorenones, or the lateral position on the methyl group resulting in a six-membered ring.3,4 The factors affecting the selectivity of competing remote metalation and lateral metalation are not fully understood. Previous experiments from our group using VT 1H NMR, EXSY NMR and some computational studies have dismissed rotational energy barriers among atropisomers as important for the selectivity.5 New experiments using larger substrates (methylnaphthyl)chrysenecarboxamides gave access to fluorescent compounds (Figure). Depending on the position of DMG and substitution pattern on naphthalenes, the strategy can be used to build the aromatic chain in suitable directions.
References:
1. Tilly, D. Magolan, J. Mortier, J. Chem. Eur. J. 2012, 18, 3804.
2. Wang, X. Fu, J‐m. Snieckus, V. HCA, 2012, 95, 2680.
3. Jørgensen, K. B. Rantanen, T. Dörfler, T. Snieckus, V. J. Org. Chem., 2015, 80, 9410.
4. Fu, J-m. Snieckus, V. Can. J. Chem. 2000, 78, 905.
5. Lorentzen, M. Kalvet, I. Sauriol, F. Rantanen, T. Jørgensen, K. B. Snieckus, V. J. Org. Chem., 2017, 82, 7300.