Dual-catalytic transition metal systems for functionalization of unreactive sites of molecules
Pawel Dydio
ISIS, University of Strasbourg, CNRS, France
Catalytic functionalization reactions occur readily at sites of starting materials that are both innately reactive and sterically accessible or that are predisposed by a functional group capable to direct a catalyst – ‘a directing group’. However, selective reactions at unbiased sites of substrates remain challenging and typically require additional pre-activation or directing group installation steps, or the use of highly reactive reagents. Therefore, the synthetic methodologies enabling for direct and selective functionalization of typically unreactive sites are highly desired.
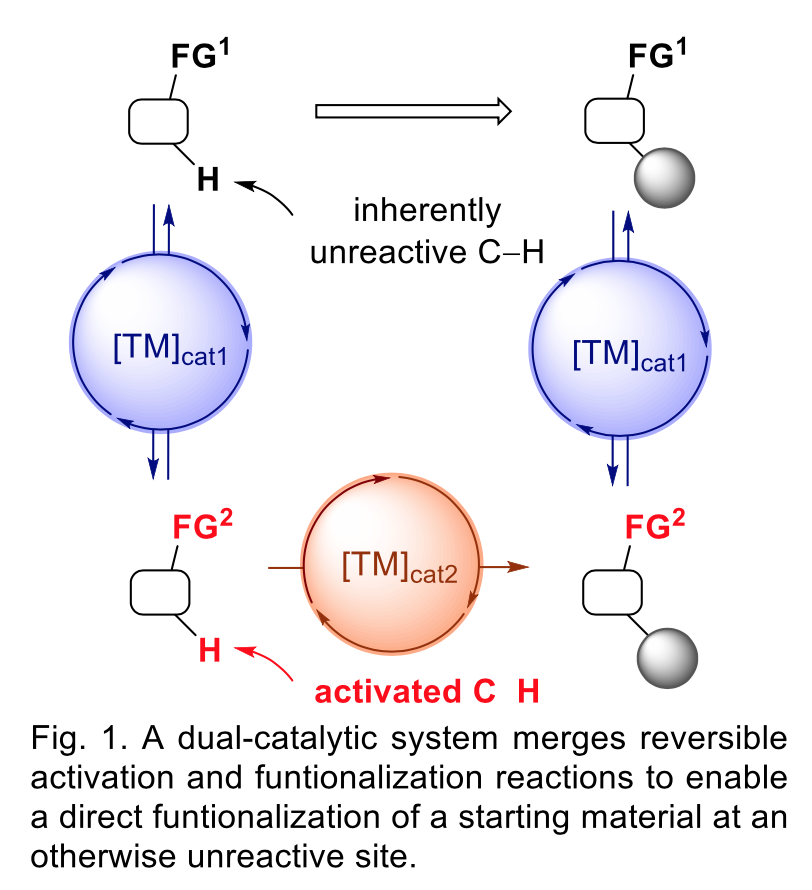
In this talk I will present our studies dedicated to the development of dual-catalytic systems that enable selective functionalization reactions of substrates at their unreactive sites, such as ubiquitous unactivated C-H bonds. Such reactions occur directly under mild conditions without the pre-activation steps and without the use of highly reactive reagents. Our strategy rests on merging a metal-catalyzed reversible reaction and a metal-catalyzed functionalization reaction (Fig. 1). For instance, we demonstrated the transient and reversible oxidation of an alcohol group by catalytic Ru-complexes enables direct arylation of the otherwise unreactive b-C-H bond of aliphatic alcohols by a Pd-catalyst, and direct g-regio- and enantioselective hydroarylation of the C=C double bond of allylic alcohols by a Rh-catalyst. Due to the mild reagents and conditions of both the reversible reaction and the functionalization reaction, the devised methodologies are general and compatible with a broad scope of substrates, including natural product-like molecules. These studies highlight the potential of the multi-catalytic approach to address challenging transformations to circumvent multi-step procedures and use of highly reactive reagents in organic synthesis.
Our current efforts in the exploration of the full potential of the approach will be further discussed.
Reference: D. Lichosyt, Y. Zhang, K. Hurej, P. Dydio, Nature Catal. 2019, 2, 114-122.
